Abstract
Aims: A combination of oral decitabine (DAC), a drug normally parenterally administered, with an oral cytidine deaminase inhibitor (CDAi), enhances its bioavailability. An oral version of DAC would provide significant patient convenience and potentially improve adherence to treatment. We previously reported results of a Phase 1 dose escalation study showing that the combination of oral cedazuridine (CED), a CDAi, at 100 mg/d and oral DAC at 30 and 40 mg/d achieved 5-day decitabine AUC levels that were 85 and 144% respectively of the 5-day AUC of DAC at 20 mg/m2 administered as a one hour intravenous infusion dailyx5 (IV-DAC). (Garcia-Manero. Blood 2016 128:114). We report here the results of a phase 2 pharmacokinetic (PK) study designed to confirm that the fixed dose combination of oral DAC (35 mg/d) and CED (100 mg/d) (ASTX727) is comparable to IV-DAC in term of DAC AUC exposure.
Methods: Adult patients with intermediate (Int) or high risk (HR) MDS or Chronic Myelomonocytic Leukemia (CMML) were enrolled in a cross-over Phase 2 study. Patients were randomized 1:1 to receive in the first 28 day cycle, either 5 days of IV-DAC or 5 days of ASTX727, followed by a cross-over to the other in Cycle 2. Cycles 3 forward were with ASTX727. Full 5-day AUC exposures of ASTX727 and IV-DAC were estimated for each patient by a model using observed data and the assumption that steady-state was achieved on Day 2. Safety and clinical response were assessed in all patients.
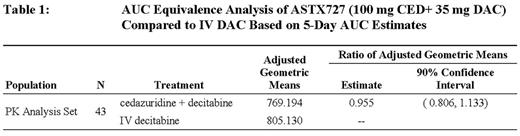
Results: 50 patients were randomized, of whom 43 had matched PK for the first 2 cycles. All patients were part of the safety and response analysis, those with matched samples were compared for the PK analysis. The median age was 69.7 yr (range 32-87), 41 (82%) were male, and 47 (94%) were previously unexposed to hypomethylating agents. The MDS-IPSS status of the patients was Int-1 in 20 (40%), Int-2 in 13 (26%) and HR in 8 (16%); 9 (18%) had CMML. The DAC AUC results from both IV-DAC and ASTX727 are presented in Table 1. Treatment with ASTX727 achieved 5-day DAC AUC which was 0.96 of that from IV DAC (90% CI 0.806 - 1.133). The median number of cycles administered were 6.5 (range 1-17). As of July 23, 2017, 17 patients remain on treatment. The most common > Grade 3 adverse events (AE) regardless of causality were neutropenia 48%, febrile neutropenia 38%, thrombocytopenia 38%, anemia 22%, leukopenia 20%, and pneumonia 20%. Only two GI AE greater than Grade 2 were observed, nausea and diarrhea, neither of which was related to treatment. Clinical benefit was observed in 31 (62%) patients, with 8 (16%) CR, 14 (28%) mCR, and 9 (18%) HI.
Summary/Conclusion: ASTX727, an oral fixed dose combination of 35 mg DAC and 100 mg CED, emulates the AUC of IV-DAC achieved with 5-day treatment at a 20 mg/m2 dose. The safety profile is similar to that reported for IV-DAC and no increase in GI side effects was observed. Response rates are also consistent with previous reports. ASTX727 will be further evaluated in a Phase 3 clinical study.
Roboz: AbbVie, Agios, Amgen, Amphivena, Array Biopharma Inc., Astex, AstraZeneca, Celator, Celgene, Clovis Oncology, CTI BioPharma, Genoptix, Immune Pharmaceuticals, Janssen Pharmaceuticals, Juno, MedImmune, MEI Pharma, Novartis, Onconova, Pfizer, Roche Pharmace: Consultancy; Cellectis: Research Funding. Busque: Pfizer: Honoraria; Paladin: Honoraria; Novartis Canada Inc.: Honoraria; Bristol Myer Squibb: Honoraria. Wells: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees. Odenike: Pfizer: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; CTI/Baxalta: Membership on an entity's Board of Directors or advisory committees; Jazz: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria. Steensma: Takeda: Consultancy; Incyte: Equity Ownership; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; H3 Biosciences: Consultancy; Onconova: Consultancy; Pfizer: Consultancy; Celgene: Consultancy. Yee: Celgene Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncoethix: Research Funding; Karyopharm: Research Funding; Novartis Canada: Honoraria; Astex: Research Funding. Faderl: Celator/Jazz: Employment, Equity Ownership. Michaelis: Novartis: Other: Consultation for New Product; Celgene: Speakers Bureau; Incyte: Other: consultation for product. Kantarjian: Novartis: Research Funding; Bristol-Meyers Squibb: Research Funding; ARIAD: Research Funding; Pfizer: Research Funding; Amgen: Research Funding; Delta-Fly Pharma: Research Funding. Oganesian: Astex Pharmaceuticals, Inc.: Employment. Lowder: Astex Pharmaceuticals, Inc.: Employment. Azab: Astex Pharmaceuticals, Inc.: Employment. Savona: Sunesis: Research Funding; Astex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Consultancy, Equity Ownership; Amgen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Consultancy, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal